Dental implants are made of medical grade titanium. This metal is inert to the natural tissues of the body, does not cause oxidation and other negative reactions. But in the manufacture of titanium alloys, a small percentage of impurities is allowed, and each element has its own electrochemical potential and bioactivity. When installing implants of different brands, metals-impurities in the acidic environment of the mouth may not combine, causing allergies, galvanic syndrome (“battery effect” in the mouth), rejection of the design.
Materials for implants
To create implants, metals not subject to corrosion, electrochemical reactions, neutral to the surrounding tissues are used. This is titanium and its alloys – a strong, lightweight material, resistant to corrosion, bioinert, contributing to the rapid formation of bone around the implant.
Manufacturers use different grades of titanium for implant constructions. In Russia they use technically pure titanium of VT 1-0 and VT 1-00 grades (GOST 19807-91). Foreign manufacturers use medical titanium of four grades (Grade 1-4 ASTM, ISO) and titanium alloy Ti-6Al-4V (ASTM, ISO) – analog of Russian VT-6. The materials differ in mechanical qualities and chemical structure.
What is “medical” titanium
Purified “medical” titanium is a non-magnetic, light, ductile metal, easily amenable to all types of mechanical processing (grinding, milling, etc.). Titanium structures are bioinert, quickly fuse with muscle and bone tissue. Titanium is chemically indifferent to all other metals. Since pure titanium is a soft material, manufacturers use impurities to give it strength.
What impurities are added to titanium and for what purpose
The main component of titanium alloys is titanium itself, to which alloying elements are added that give the alloys different properties. They use:
- zirconium;
- aluminum;
- vanadium;
- molybdenum;
- iron;
- cobalt;
- nickel;
- chromium;
- copper;
- silicon, etc.
Alloying additives make the strength of titanium alloy higher and have other features. For example: zirconium is bioinert and is considered a neutral hardener for titanium. Structures made of this alloy are characterized by high biocompatibility, increased mechanical properties. Chromium, nickel or vanadium are added to increase strength, reduce hydrogen embrittlement of the alloy. The addition of these alloyed components makes the alloy cheaper. But given the likelihood of developing a severe allergy to the same nickel, it is not even worth talking about saving money.
The danger of inexpensive impurities
Some manufacturers use titanium alloys containing vanadium, chromium, nickel for dental implants. Mechanical qualities are obtained excellent, but they are toxic to the surrounding tissues, causing allergic reactions. Artificial roots with these metals in the composition, characterized by a lower degree of engraftment. Inclusion of alloying impurities, contributes to the formation of connective tissue layer around the implant and contamination of tissues, which leads to peri-implantitis, rejection of the design.
What materials are used in the ROOTT Clinic
For the manufacture of implant structures, suprastructures, prosthetic arches and ROOTT prosthetics, titanium grade 5 (Ti Grade 5) is used.
The advantages of the material are:
- Biocompatibility;
- absence of toxic impurities (vanadium, nickel, etc.);
- increased resistance to corrosion;
- quick integration with fabric;
- absence of allergies.
Grade 5 titanium is referred to as “commercially pure”. It does not cause an aggressive reaction from the immune system, quickly integrates with the jawbone. The quality of the material is confirmed by a certificate of conformity.
In the clinic, titanium is used not only for implants, it is also used for the metal base of orthopedic constructions. It is inadmissible to use nickel-chromium alloy for prosthetic arches. Nickel is a powerful allergen and has a toxic effect on the human body. It is allowed to use a metal alloy of cobalt-chromium, but only in the absence of allergies. In our clinic, to avoid complications in the form of allergic reactions, all products are made only of medical titanium.
ROOTT products are certified and authorized for use in the Russian Federation. Registration certificates are issued by the order of Roszdravnadzor. Declarations of conformity of Trate AG products and instruments to the requirements of approved GOSTs based on clinical tests of products have been issued.
Results of ROOTT implants surface quality assessment
The functioning period of a dental implant depends on the biocompatibility of the implant construction material, strong bone and fibrous-bone connection. The surface structure of the titanium root affects the formation of a reliable contact with the jawbone and the distribution of stress in the jaw under masticatory load.
The reliability and durability of intraosseous implant fixation is achieved by increasing the contact area by processing the surface of the artificial root (sandblasting, acid etching or a combination of both). Due to the manufacturing process, foreign particles that are not part of the structure of the material from which the implant is made may settle on the surface of the implant.
According to the results of an analysis of different brands of implants conducted in 2015 by the Institute for Medical Materials Research (Berlin). Institute for Medical Materials Research (Berlin). Out of 135 samples, the Roott implant (TRATE AG) is the only implant design with no contaminants of organic or inorganic origin found on its surface. The combinatorial method of sandblasting with hydroxyapatite/tricalcium phosphate microparticles leaves no traces on the implant surface.
What are the dangers of contaminants on the implant surface?
The manufacturer supplies dental implants in sterile packaging, which implies that their surface is clean. Contamination of the surface of the artificial root with chromium, copper, iron, tin, stainless steel particles causes partial or complete lack of osseointegration.
The fusion of the artificial root with the bone (osseointegration) is not a static, but a dynamic process. It begins immediately after surgical placement of the titanium structure. Random microscopic particles of organic origin with traces of chromium, nickel or antimony can provoke an immune reaction to a foreign body, which will end in peri-implantitis and rejection of the implant.
What are the dangers of implants made of different materials?
Some doctors, in order to make the implantation process cheaper, offer the patient a combination of implants according to the principle – “some are more expensive, others are cheaper”. For example, in case of complete adentia, two Nobel implants out of six implants are installed, and the other four are Biomed. This is a categorically wrong approach. When installing implants from different manufacturers, metals-impurities under the influence of the acidic environment of the oral cavity, can come into conflict, which will provoke the development of serious complications:
- allergic reaction;
- galvanic syndrome;
- peri-implantitis with implant rejection.
The surface of cheaper implants is contaminated with metal microparticles, which are not part of the material structure, but are formed in the process of processing the surface of the structure. The presence of such contaminants negatively affects the process of osseointegration.
Installing orthopedic systems with metal components or metal-ceramic crowns made of other alloys, add another type of metal to the oral cavity, which further worsens the situation.
What is galvanic effect
In the presence of structures made of different types of metals, under the influence of an acidic environment, electric potential values change and a current of different strengths is generated. This phenomenon is called galvanism. Galvanic syndrome (galvanosis) is a pathological condition that occurs in the oral cavity due to the effect of galvanic currents on the surrounding tissues. The disease is manifested by the following symptoms:
- metallic taste in the mouth;
- a persistent bitterness;
- tingling and burning of the tongue;
- dry mouth;
- changes in taste sensations;
- numbness or hypersensitivity of the oral mucosa;
- itching in the gums;
- sensation of “flowing” current across the mucosa;
- sleep disturbance;
- decreased immunity;
- frequent headache;
- rapid fatigue;
- decreased efficiency.
The probability of developing a pathological condition is multiplied in patients prone to allergic reactions.
Complications
If the metal elements that provoked galvanosis are not removed, it can lead to the development of serious problems:
- gingivitis;
- papillitis;
- stomatitis;
- allergies;
- leukoplakia;
- other precancerous and malignant conditions of the oral mucosa.
Treatment of galvanosis is to eliminate the cause of its development – identification and removal of problematic metal components (dissimilar metals, structures with signs of corrosion). Sanitation of the oral cavity, treatment of detected diseases, immunocorrection. A complete re-prosthetics is performed with constructions of homogeneous material, which excludes the occurrence of galvanism.
What is fraught for the patient with the disease
Treatment of galvanosis involves the removal of all metal structures, including implants. Complete re-prosthetization is fraught with additional financial and time expenditures, and not insignificant:
- After the removal of the implant, it will be necessary to restore the volume of bone tissue. This involves surgery to build it up (osteoplasty), rehabilitation period and about 6 months for grafting of the bone-plastic material, restoration of bone.
- Installation of new implants (and time for their engraftment), orthopedic system.
Since implantation does not belong to the cheap methods of dental prosthetics, the patient will not be happy with this treatment option. Therefore, it is necessary to exclude even a minimal probability of the development of the “battery effect”.
Precautions
- Installation of implants, suprastructures, orthopedic constructions with metal components of homogeneous material.
- Choose a clinic that can provide certificates for all materials and components used.
- Install implants from the same manufacturer. Some clinics offer to select designs of different brands to reduce the cost of treatment. However, such savings threatens even greater financial costs. Do not agree in any case.
- Install crowns made of hypoallergenic, bioinert materials – precious metals, zirconium oxide, not causing oxidation, other negative reactions.
- It is obligatory to inform the doctor about the presence of metal structures (pins, inlays, endoprostheses) already installed in the oral cavity.
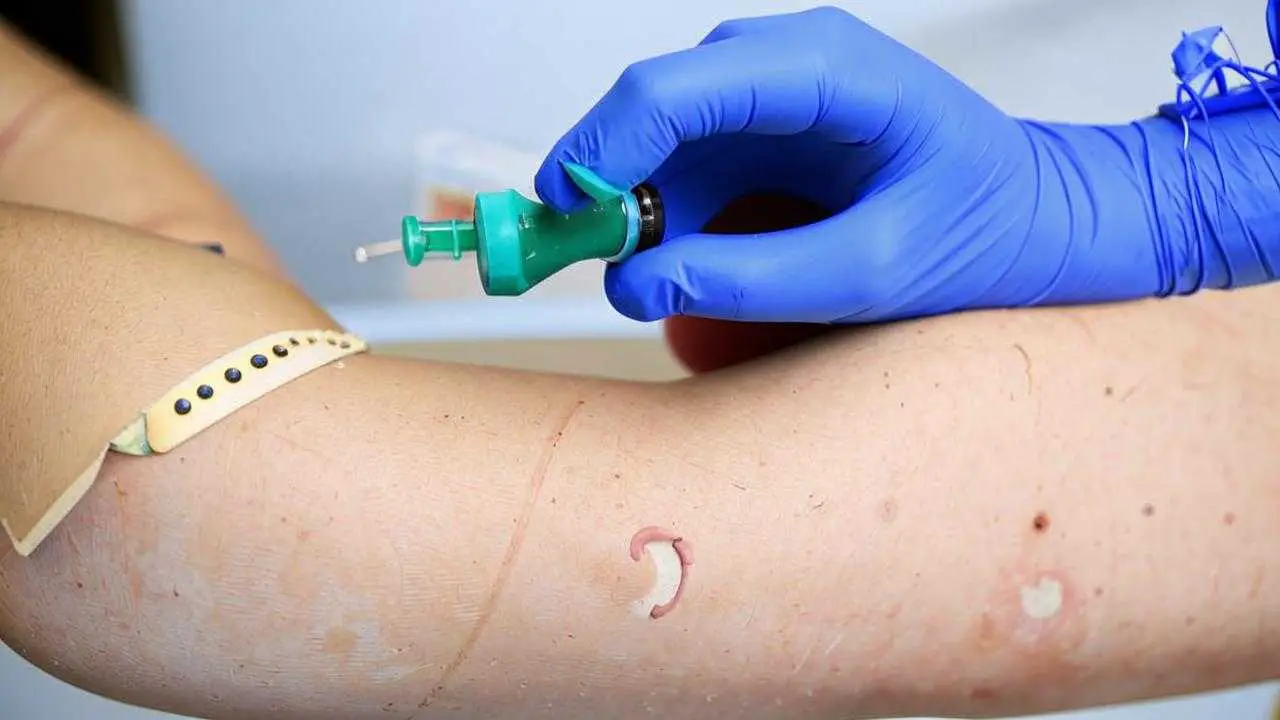
Why allergy tests are necessary
If the patient has doubts about the tolerance of metal, it is recommended to perform an allergy test before the implant and orthopedic construction are placed. Allergy tests for components present in the alloy can be performed in a laboratory.
Indications for testing are the presence in the patient’s history:
- Previously installed metal structures (pins, inlays, orthopedic systems).
- Allergic diseases (atopic or contact dermatitis, bronchial asthma, seasonal allergies).
- Atypical reactions to materials used in dentistry (metals, plastics and alloys).
- Chronic diseases of the oral cavity, digestive system.
- Immune disorders.
To detect allergic reactions, the following methods are used:
- Blood tests for specific immunoglobulin IgE and IgG in serum;
- Skin tests (scarification, drop tests) for nickel, chromium and other metals.
To analyze the reaction, the ROOTT clinic provides the patient with the material from which implants, suprastructures (abutments, gum shapers), orthopedic systems are made.
Advantages of implantation at ROOTT
- All dental structures are made of grade 5 titanium.
- Sandblasting with hydroxyapatite/tricalcium phosphate particles eliminates the presence of foreign impurities on the implant surface.
- The quality of the material is confirmed by a certificate of conformity.
- All ROOTT products are certified and authorized for use in our country.
- ROOTT implant systems are represented by different models, which allows to choose implants of one brand for any clinical situation without resorting to products of other manufacturers.
- The development of galvanic syndrome is excluded.
- For allergic reaction tests, the patient is provided with the material from which the applied structures are made.